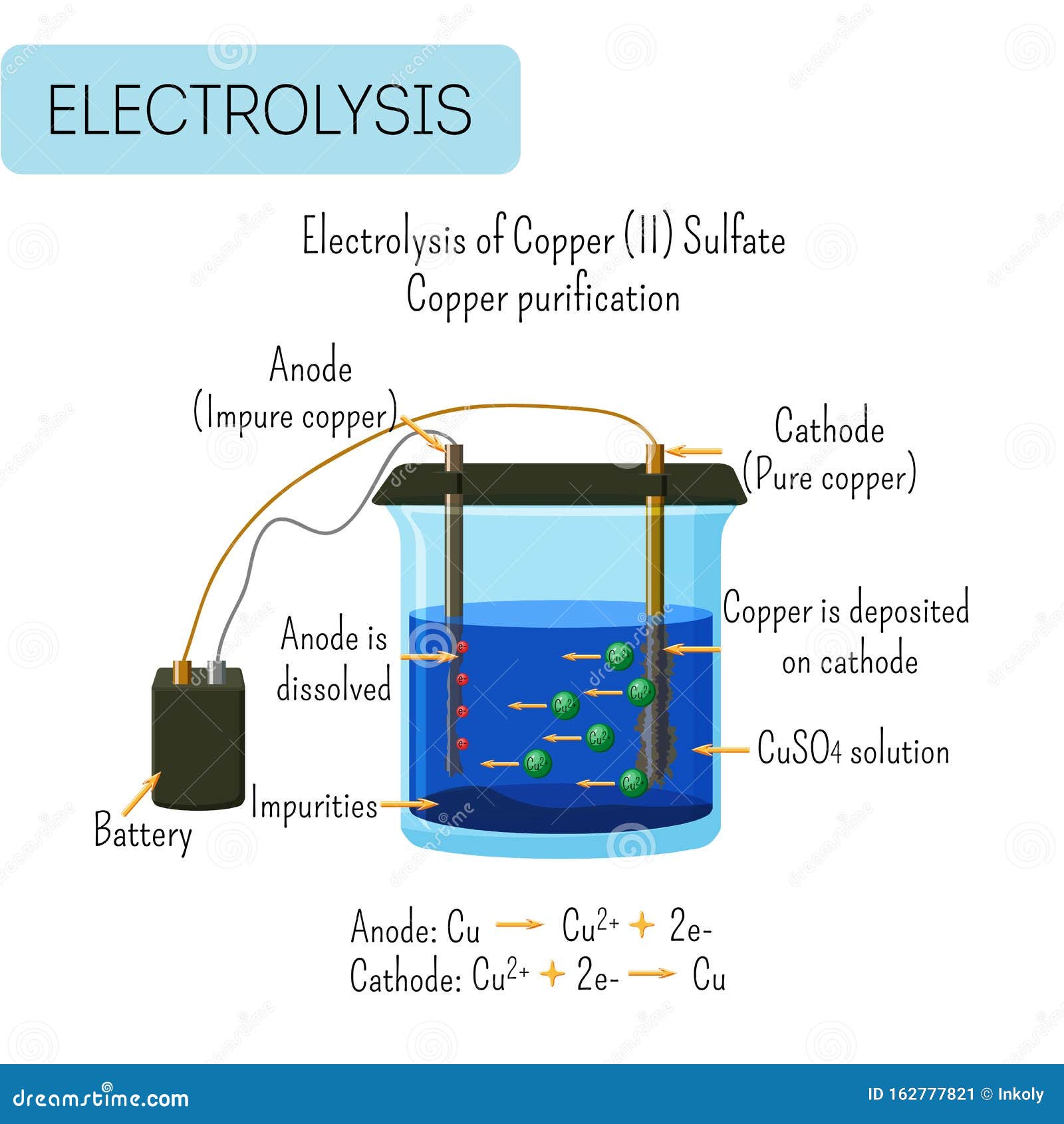
Copper Electroplating Copper Sulfate . in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source of copper for electroplating. The reaction is the reverse of the cathode reaction. in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. You can carry out the process in copper sulfate electrolysis. Sulfuric acid (h2so4) makes the bath conductive. if copper is used for the electrodes, the copper anode dissolves. the concentration of copper sulfate helps determine the properties of the baths. copper electroplating is coating a thin copper film on a metal substrate. copper sulfate (cuso4) provides a source of copper ions. The results of this experiment can lead to a.
from www.dreamstime.com
in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. the concentration of copper sulfate helps determine the properties of the baths. in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source of copper for electroplating. You can carry out the process in copper sulfate electrolysis. copper electroplating is coating a thin copper film on a metal substrate. copper sulfate (cuso4) provides a source of copper ions. Sulfuric acid (h2so4) makes the bath conductive. in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. if copper is used for the electrodes, the copper anode dissolves. The results of this experiment can lead to a.
Electrolysis of Copper Sulfate Solution with Impure Copper Anode and
Copper Electroplating Copper Sulfate copper electroplating is coating a thin copper film on a metal substrate. You can carry out the process in copper sulfate electrolysis. in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. the concentration of copper sulfate helps determine the properties of the baths. copper electroplating is coating a thin copper film on a metal substrate. if copper is used for the electrodes, the copper anode dissolves. in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source of copper for electroplating. The results of this experiment can lead to a. Sulfuric acid (h2so4) makes the bath conductive. in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. copper sulfate (cuso4) provides a source of copper ions. The reaction is the reverse of the cathode reaction.
From www.exportersindia.com
Copper Sulphate Electroplating Grade, for Dies, Color Blue at Rs 210 Copper Electroplating Copper Sulfate in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. The reaction is the reverse of the cathode reaction. if copper is used for the electrodes, the copper anode dissolves. in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source. Copper Electroplating Copper Sulfate.
From www.embibe.com
How can you electroplate an iron nail with copper Explain with the help Copper Electroplating Copper Sulfate Sulfuric acid (h2so4) makes the bath conductive. You can carry out the process in copper sulfate electrolysis. copper sulfate (cuso4) provides a source of copper ions. the concentration of copper sulfate helps determine the properties of the baths. The results of this experiment can lead to a. if copper is used for the electrodes, the copper anode. Copper Electroplating Copper Sulfate.
From www.lazada.com.my
1000G 98 sulphuric acid industrial grade CAS7758 99 8 electroplating Copper Electroplating Copper Sulfate in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. if copper is used for the electrodes, the copper anode dissolves. copper sulfate (cuso4) provides a source of copper ions. The results of this experiment can lead to a. in its most. Copper Electroplating Copper Sulfate.
From www.mdpi.com
Micromachines Free FullText Study of Copper Electrodeposition at a Copper Electroplating Copper Sulfate in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. copper sulfate (cuso4) provides a source of copper ions. Sulfuric acid (h2so4) makes the bath conductive. the concentration of copper sulfate helps determine the properties of the baths. copper electroplating is coating a thin copper film on. Copper Electroplating Copper Sulfate.
From www.mdpi.com
Materials Free FullText Effect of Copper Sulfate and Sulfuric Acid Copper Electroplating Copper Sulfate You can carry out the process in copper sulfate electrolysis. copper electroplating is coating a thin copper film on a metal substrate. in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source of copper for electroplating. The results of this experiment can lead to a. if copper is used. Copper Electroplating Copper Sulfate.
From runxuchen.en.made-in-china.com
Copper Sulfate Industrial Grade CAS 7758998 for Tanning and Copper Copper Electroplating Copper Sulfate copper sulfate (cuso4) provides a source of copper ions. copper electroplating is coating a thin copper film on a metal substrate. in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. the concentration of copper sulfate helps determine the properties of the. Copper Electroplating Copper Sulfate.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Copper Electroplating Copper Sulfate Sulfuric acid (h2so4) makes the bath conductive. copper electroplating is coating a thin copper film on a metal substrate. the concentration of copper sulfate helps determine the properties of the baths. The reaction is the reverse of the cathode reaction. copper sulfate (cuso4) provides a source of copper ions. in this deep dive, we'll explore how. Copper Electroplating Copper Sulfate.
From www.youtube.com
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes YouTube Copper Electroplating Copper Sulfate copper sulfate (cuso4) provides a source of copper ions. in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. The results of this experiment. Copper Electroplating Copper Sulfate.
From www.youtube.com
Easy StepbyStep Tutorial on Electroplating a CopperPlated Key YouTube Copper Electroplating Copper Sulfate Sulfuric acid (h2so4) makes the bath conductive. the concentration of copper sulfate helps determine the properties of the baths. copper electroplating is coating a thin copper film on a metal substrate. The reaction is the reverse of the cathode reaction. You can carry out the process in copper sulfate electrolysis. The results of this experiment can lead to. Copper Electroplating Copper Sulfate.
From www.shutterstock.com
Electroplating Copper Using Copper Sulfate Electrolyte Stock Vector Copper Electroplating Copper Sulfate in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. The results of this experiment can lead to a. You can carry out the process in copper sulfate electrolysis. if copper is used for the electrodes, the copper anode dissolves. copper electroplating is. Copper Electroplating Copper Sulfate.
From www.nagwa.com
Question Video Identifying the Setup Appropriate for Electroplating Copper Electroplating Copper Sulfate copper electroplating is coating a thin copper film on a metal substrate. the concentration of copper sulfate helps determine the properties of the baths. The results of this experiment can lead to a. in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. in this deep dive,. Copper Electroplating Copper Sulfate.
From www.verywellhealth.com
Copper Sulfate Benefits, Side Effects, Dosage, and Interactions Copper Electroplating Copper Sulfate in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. copper sulfate (cuso4) provides a source of copper ions. in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source of copper for electroplating. in this deep dive, we'll explore. Copper Electroplating Copper Sulfate.
From xtdktech.en.made-in-china.com
Copper Sulfate for Feed / Agriculture / Electroplating / Fertilizer Copper Electroplating Copper Sulfate the concentration of copper sulfate helps determine the properties of the baths. copper electroplating is coating a thin copper film on a metal substrate. copper sulfate (cuso4) provides a source of copper ions. if copper is used for the electrodes, the copper anode dissolves. The reaction is the reverse of the cathode reaction. in addition. Copper Electroplating Copper Sulfate.
From www.youtube.com
electroplating experiment using copper sulphate solution, copper metal Copper Electroplating Copper Sulfate copper electroplating is coating a thin copper film on a metal substrate. copper sulfate (cuso4) provides a source of copper ions. if copper is used for the electrodes, the copper anode dissolves. in addition to facilitating the transfer of electrons (electrical flow), the sulfate solution also acts as a source of copper for electroplating. The reaction. Copper Electroplating Copper Sulfate.
From www.lazada.com.my
1000G 98 sulphuric acid industrial grade CAS7758 99 8 electroplating Copper Electroplating Copper Sulfate in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. if copper is used for the electrodes, the copper anode dissolves. the concentration of copper sulfate helps determine the properties of the baths. copper electroplating is coating a thin copper film on a metal substrate. Sulfuric acid. Copper Electroplating Copper Sulfate.
From www.youtube.com
Timelapse of copper sulfate electrolysis YouTube Copper Electroplating Copper Sulfate Sulfuric acid (h2so4) makes the bath conductive. copper sulfate (cuso4) provides a source of copper ions. You can carry out the process in copper sulfate electrolysis. The results of this experiment can lead to a. in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. copper electroplating is. Copper Electroplating Copper Sulfate.
From www.indiamart.com
Copper Sulphate Electroplating Grade, Powder, Pandora Industries Copper Electroplating Copper Sulfate copper electroplating is coating a thin copper film on a metal substrate. Sulfuric acid (h2so4) makes the bath conductive. The results of this experiment can lead to a. in this deep dive, we'll explore how copper electroplating works, how you can do it at home, the materials you'll need for a lab. in its most basic form,. Copper Electroplating Copper Sulfate.
From brainly.in
how can you electroplate an iron nail with copper. explain with the Copper Electroplating Copper Sulfate You can carry out the process in copper sulfate electrolysis. the concentration of copper sulfate helps determine the properties of the baths. Sulfuric acid (h2so4) makes the bath conductive. in its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis. in this deep dive, we'll explore how copper electroplating. Copper Electroplating Copper Sulfate.